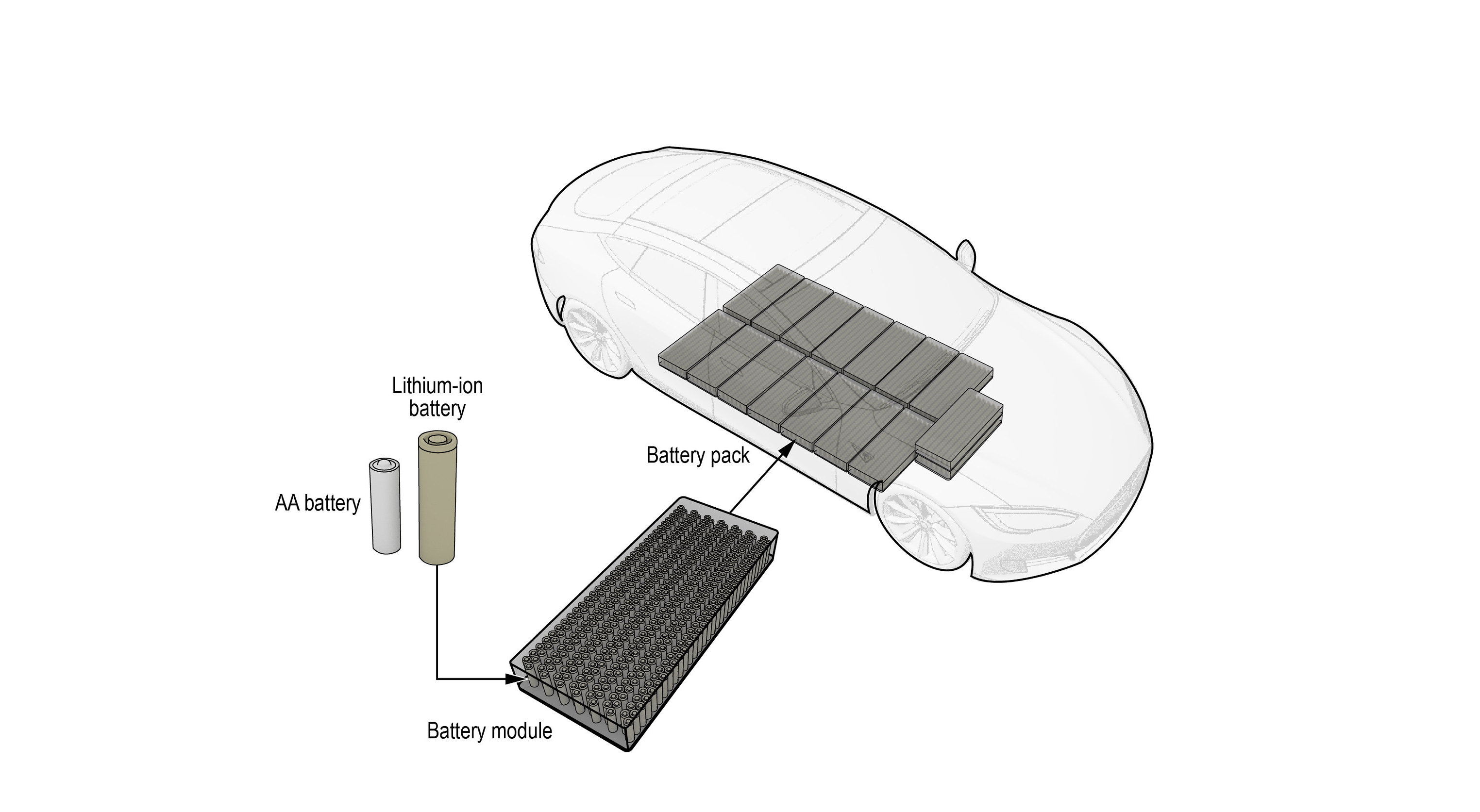
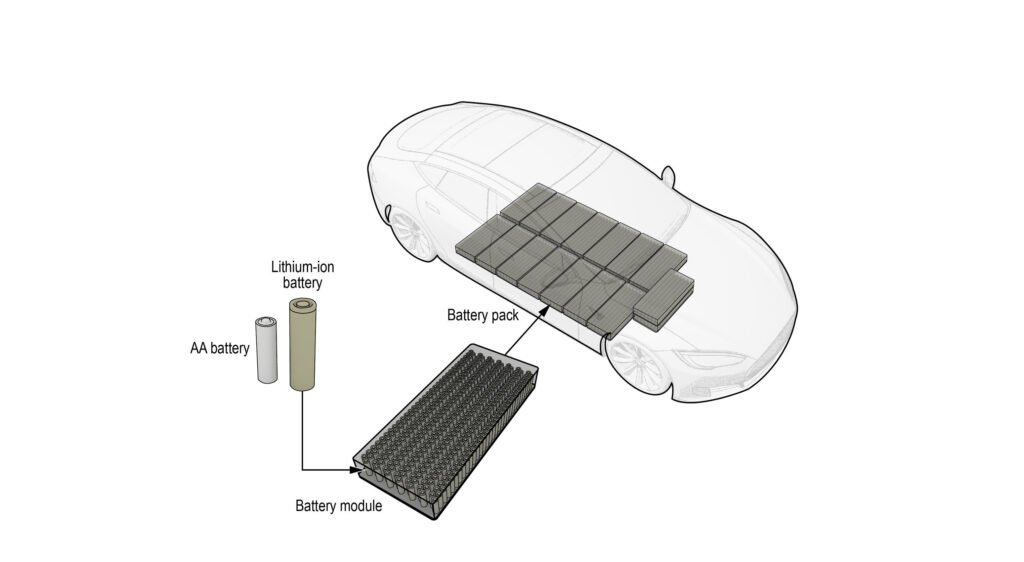
The lithium-ion batteries found in EVs, smartphones, headphones, and many other gadgets use a reversible chemical reaction to circulate electrons and generate electrical energy.
Lithium-ion batteries are present in most of your high-tech rechargeable devices such as smartphones, tablets, laptops, speakers, headphones, etc. They play a central role in the modern mobile culture as they fuel most of the technologies that we use every day.
Also commonly called “li-ion”, lithium-ion battery technology has enabled the rise of electric vehicles, whether we’re talking about electric cars, electric motorcycles, or micromobility vehicles like skateboards and scooters.
Li-ion battery cells are also present in residential energy storage systems like Tesla Powerwall, but also utility-scale deployments that store energy generated by fluctuating sources like wind and solar power stations.
Table of Contents
How do lithium-ion batteries work?
Any battery consists of multiple individual battery “cells” that are connected to each other. In turn, each battery cell consists of three main elements: a positive electrode (cathode), a negative electrode (anode), and a liquid electrolyte.
Much like the alkaline dry cells used in things like remote controls, lithium-ion batteries create energy through the movement of ions.
In its elemental form, lithium is extremely reactive. This is the reason elemental lithium is not used in lithium-ion batteries. Instead, the cathode contains a metallic lithium salt, such as lithium cobalt oxide (LiCoO2). This is what supplies lithium ions to the system.
So called reduction-oxidation reactions take place in a lithium-ion battery. The energy stored in the battery is released as the lithium ions travel through the electrolyte between the positive and negative electrodes. When the li-ion battery is recharged, the opposite reaction takes place, with electrons moving in the opposite direction.

Where is lithium-ion tech used?
Lithium-ion batteries are used in multiple applications, but the most common use is in the electronics industry. They are commonly used to power portable electronics such as mobile phones, tablets, and laptops. Other uses for these batteries include electric vehicles, power tools, and medical equipment.
Also read: 10 alternatives to lithium-ion batteries: Which new tech will power the future?
What are the benefits of lithium-ion batteries?
- Low maintenance – there is no need to discharge these batteries periodically.
- There’s no need for prolonged priming when the batteries are new, like it happened with older types of battery tech. You can simply charge a device using a lithium-ion battery regularly and use it straight out of the box.
- Lithium-ion batteries have a relatively low self-discharge, which is less than a half than that of nickel batteries. That means they don’t lose a lot of power when they sit unused for a while.
- They offer high energy density, with the potential for even higher capacity with future development. Li-ion can store a lot of energy relative to their size.
What are the limitations of lithium-ion?
- Lithium-ion batteries are about 40% more expensive to manufacture than nickel-cadmium batteries.
- Li-ion batteries age relatively rapidly, even when not in use. In some cases, a capacity drop is noticeable after just one year, even if the battery is not in use. That’s the reason gadgets tend to lose “battery life” after being charged and discharged repeatedly over the course of their lifetime.
- There are some transportation restrictions for shipments of lithium-ion batteries. Airlines commonly require the transport of spare li-ion batteries in carry-on luggage, to minimize risks caused by potential fires.
- Lithium-ion batteries require a protection circuit to keep current and voltage within safe limits.
Why do lithium-ion batteries degrade?
Lithium-ion batteries are susceptible to damage when they’re charged beyond their voltage limits, but they can also degrade prematurely when stored for too long.
This effect is a result of the chemistry of the battery, as unavoidable chemical reactions take place inside it not only during runtime but also when not in use. These reactions make it impossible for lithium-ion batteries to maintain their full capacity throughout their lifetime.

What are some of the downsides of lithium-ion batteries?
Lithium-ion batteries have very impressive characteristics. However, they also have disadvantages. First of all, they have limited lifespans, even if they are not used. A typical lithium-ion battery lasts only two to three years. Such a frequency of replacement involves significant costs. In addition, the production and disposal of lithium-ion batteries have a great impact on the environment.
Because lithium is extremely reactive, manufacturers need to take precautions so that the public can use lithium-ion batteries safely. However, you may have heard of electronic devices, such as laptops or mobile phones, which have caught fire due to faulty batteries. For safety reasons, lithium-ion batteries have a built-in separator so the electrodes don’t touch each other. But if the separator is torn or damaged, contact between the electrodes can cause a substantial build-up of heat. And if this heat build-up creates a spark the electrolyte can catch fire.
Also read: Regenerative braking explained: What is it and how does it work?
However, you don’t need to worry too much as such situations are extremely rare. Lithium-ion batteries are actually very safe. In addition, research continues to improve each component of these batteries. For example, researchers have created a liquid electrolyte that turns into a solid when impacted. This will make the batteries less likely to overheat or catch fire if damaged.